Have you ever considered that for your novel food ingredient, the much faster access to the European Union (EU) market is to sell it first as feed material or cosmetic ingredient? When you start making money from these markets, you will have the resources to prepare a novel food application. In my current blog, I will reflect on this question – a question which is not often considered by novel food developers.
EU Golden Food Safety Standard
We all know that the route to the EU’s novel food markets is tedious and long. We are well aware of the respective EU novel food regulations, the detailed regulatory guidance documents by the European Food Safety Authority (EFSA), and the roles of the European Commission (EC) and all 27 Member States (MSs) in making the final market-access decisions for novel foods.
On the other hand, we need always to remember that we, as European food consumers and voters, have wanted to have this high-level food safety system to be able to eat safe food! The EU food safety system is often regarded as the “golden standard” worldwide. A part of this golden standard is naturally the feed safety system, but luckily, feed regulations do not have a similar ‘novel feed’ category, while the regulatory requirements for feed additives can be seen as comparable to the requirements for food additives.
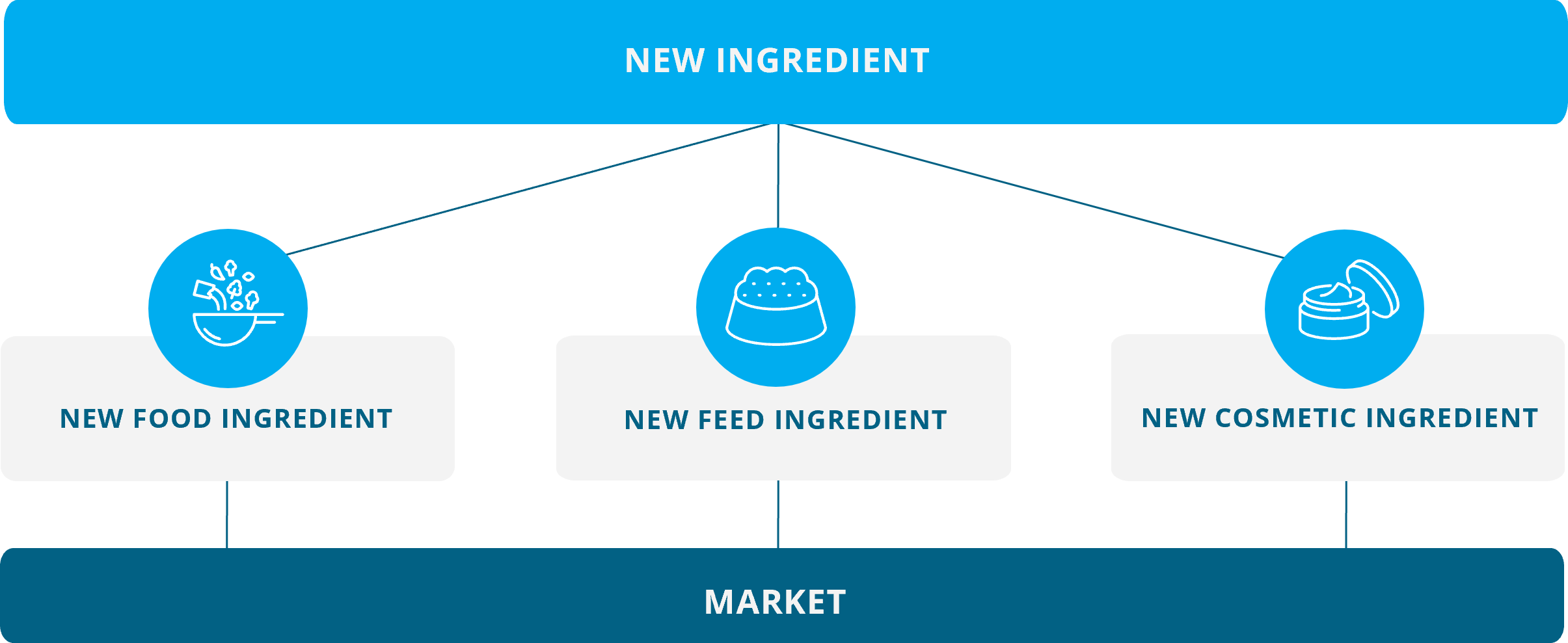
The same novel food ingredient could equally be used in feeds and cosmetic products
In the current changed world order and landscape, where climate change makes agriculture unpredictable and highly challenging, ongoing wars limit access to ingredients (e.g., Ukrainian sunflower oil or Houthis at the Red Sea), and trade wars may block the free movement of ingredients, new feed material and cosmetic ingredient sources are continuously sought after. Our livestock and pets need to be fed, and our skins require their moisturising oils to remain in good condition.
As depicted below, the same novel food ingredient used in foods could equally be used as an ingredient in feeds and cosmetic products. It is easy to understand that ingredients like proteins, fats, carbohydrates, vitamins, and antioxidants are also important components in terrestrial and aquatic livestock feeds, pet foods, and human cosmetic products.
No formal authorisation is needed for feed materials and cosmetic ingredients
Certainly, as with all foods, all feeds and cosmetics need to be safe for human/animal consumers, and this legal responsibility lies with the business operators. The major difference is, however, that for feed materials and most cosmetic ingredients, there is no formal legal authorisation process. Only for certain cosmetic ingredients, such as preservatives and hair dyes, is there an EU-level pre-market authorisation process to pass, but the typical novel food ingredients do not usually fall under these specific ingredient classes. For feed materials that have not previously been marketed in the EU, a simple registration step is needed. A similar type of notification is required for cosmetic products but not for cosmetic ingredients.
In addition, if the annual production of the cosmetic ingredient is over one ton per year, a REACH registration is mandatory. But, again, for the novel food types of ingredients, REACH can provide some exemptions. REACH stands for Registration, Evaluation, Authorisation, and Restriction of Chemicals, and the European Chemicals Agency (ECHA) in Helsinki is the responsible agency. If REACH registration is required, then a full dossier, including chemical and toxicological data, has to be submitted to ECHA.
In the EU, prior to lawful marketing, cosmetic products need to be notified by the responsible person of the products to the cosmetic products notification portal (CPNP). The EC is managing the CPNP.

Livestock feed and pet food
Animals need energy and nutrition similarly to any living organism. Feed materials provide energy and nutrition; this being their primary function. For example, proteins, carbohydrates, and fats, such as precision-fermented proteins and fats or algal press cakes, are feed materials. Naturally present vitamins, minerals, and antioxidants in feed materials are permitted as long as their function is secondary.
Following the in-house safety assessment of the feed material for the target animal, registration in the EU-feed material register is required unless it has already been registered by another feed business operator or listed in the EU’s feed material catalogue. Thereafter, the feed ingredient can be lawfully sold on the EU market as feed material. The feed material has to be safe not only for the target animal but also for the environment. Also, the safety of workers who work with the feed materials has to be considered. Consumer safety is only relevant to assess if there is any carry-over from feed material to food products. The feed business operator also needs to remember to register in the register of feed business operators at the MS-level.
Another interesting aspect of feed materials is that they are consumed by many different species, while food is consumed only by one – humans. The feed materials can be targeted for different animal species. Food-producing animals, such as dairy cows, beef cattle, poultry, pigs, goats, horses, rabbits, fish, and crustaceans, all need their feed, as do companion animals such as dogs, cats, horses, rabbits, fish, rats, guinea pigs, and ornamental birds. While feed materials must be safe for their target animals, the marked difference from foods is that, for example, the statutory maximum levels for chemical contaminants in feeds are much higher than they are in foods. Moreover, the number of required safety studies for animal feed is typically a bit lower than it is for human food due to the fact that animals do not live as long as humans.
One or several cosmetic ingredients are needed for a cosmetic product
The cosmetic product industry is also interested in new ingredients. Different oils and fats, such as precision-fermented fats and algal oils, are good ingredients to be used in moisturising cosmetic products. In addition, biologically active substances, such as vitamins and antioxidants, are also important ingredients in cosmetic products. As for feed materials, the in-house safety assessment has to be conducted for the cosmetic ingredient by the business operator before it can be sold further to the cosmetic product formulator. Since cosmetic product safety is the sum of the safety of its ingredients, the product manufacturers certainly require safety evidence for each ingredient. As in the case of feed materials, the safety of workers also has to be considered.
As regards cosmetics, an important peculiarity to remember is that experimental animal assays for demonstrating the safety of cosmetic ingredients and products are not permitted in the EU. Therefore, the relevance of chemical and physicochemical characterisation of the cosmetic ingredient is emphasised in the safety evaluation. Also, in silico, read across, and in vitro studies, and new approach methods (NAMs) are commonly applied when gathering safety evidence for cosmetic ingredients. Naturally, these assays and methodologies are much less expensive than the experimental animal studies still typically required for novel foods.
Finally, it is also good to realise that the market-access requirements for feed materials and cosmetic ingredients in the UK are very similar to the ones on the continent side of Europe.
From the income generated by selling feed and cosmetic ingredients, you may then expand your business to include novel foods
I hope this blog text made you think that there are also ways other than the novel food route to access the markets with your new ingredient. The new ingredients can provide us not only with a much-needed diversification of feed material and cosmetic ingredient sources but also income and new workplaces. These we desperately need in this economic, environmental, and political situation.
Thus, from many perspectives, the feed material and cosmetic ingredient regulatory routes are good alternatives to novel foods to access the market. What may happen over time is that after your business has got enough revenue by selling feed and cosmetic ingredients, you may then expand your business to the novel food sector.

Free Webinar: Novel food classification and route to novel food authorisation in the EU
In this practical webinar you’ll get an overview of the classification of potential novel foods, the official consultation procedure, as well as the novel food authorisation process and its requirements. We will also go through a success story of a marine novel food authorisation in which we assisted our client.
We do feed materials and cosmetic ingredients at Medfiles
Feed materials and cosmetic ingredients are important expertise areas of ours at Medfiles. Our high knowledge of novel foods also helps us while working with feed materials and cosmetic ingredients. In addition, with our novel food expertise, we are able to tailor the data required for the safety demonstration of feed materials and cosmetic ingredients in such a manner that the same data can be equally used for novel foods.
We regularly work with feed materials and also with cosmetics in clients’ cases by assisting them to find the easiest and fastest access to the markets. We do not only conduct safety assessments for different types of ingredients based on the client data but also assist the client hand in hand to identify and gather the data required for the assessments. Medfiles also assist you with the legally correct labelling of feeds and cosmetic products. Our very relevant assets in this work are long-lasting and versatile regulatory experience, expertise in human, farm, and companion animal risk assessments acquired by our experts through working at EFSA for ten years and our expertise in human toxicology and applied chemistry.
Thus, my Medfiles colleagues and I would be delighted to talk to you about your ideas and explore them further. I am positive that these discussions would be fruitful for both parties – you and the Medfiles experts.
Whenever you need assistance with the regulatory affairs of human food, animal feed, or cosmetics, we are at your service and more than happy to help you.
See also related publications:
- Free webinar: Novel food classification and route to novel food authorisation in the EU
- Free webinar: Regulatory aspects of feed labelling in the EU
- Free webinar: Overview of food labelling in the EU
- Importance of regulatory knowledge in transforming science to commercial food and feed products

Author: Dr Mari Eskola, Senior Regulatory Affairs Expert, Team Leader of Regulatory Science and Reports Team, Unit on Food & Feed and Cosmetics, Medfiles Ltd
Mari Eskola joined Medfiles in 2021. Since then, she has been involved in several projects on food, feed and cosmetics conducted for Medfiles’ clients. Mari is a food chemist specialised in analytical chemistry and in 2002, she obtained a PhD degree in food science. Mari has over 25 years of international expertise in the chemical and regulatory safety of food and feed from the European Union and European national organisations as well as from the industry. She has worked for 10 years at the European Food Safety Authority (EFSA), carrying out EFSA regulatory risk assessments of contaminants in foods and feeds, where she also worked as acting and deputy head of the EFSA Contaminants Unit.
In addition, Mari has food and feed research and regulatory expertise from various other European institutes, such as the European Commission Joint Research Centre (EC JRC), the European Chemicals Agency (ECHA), Teagasc in Ireland, the Austrian University of Natural Resources and Life Sciences and the former Finnish Food Safety Authority. Mari has international experience in managing projects and people, and she has authored several scientific publications comprising also many scientific EFSA opinions and risk assessments.
References:
Regulation (EC) No 767/2009 of the European parliament and of the Council of 13 July 2009 on the placing on the market and use of feed, amending European Parliament and Council Regulation (EC) No 1831/2003 and repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC. https://eur-lex.europa.eu/homepage.html?locale=en
Commission regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials
Commission recommendation of 14 January 2011establishing guidelines for the distinction between feed materials, feed additives, biocidal products and veterinary medicinal products. https://eur-lex.europa.eu/homepage.html?locale=en
Regulation (EC) No 1223/2009 of the European parliament and of the Council of 30 November 2009 on cosmetic products. https://eur-lex.europa.eu/homepage.html?locale=en
Scientific Committee on Consumer Safety (SCCS). 2023. The SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation, 12th revision. https://health.ec.europa.eu/latest-updates/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision-2023-05-16_en


