
The Medfiles Baltics team has served its clients for more than 25 years. During the years, we have assisted our clients with various tasks in the field of regulatory affairs and pharmacovigilance and helped them through many kinds of demanding situations. Because the three Baltic countries, Estonia, Latvia and Lithuania, are relatively small, many companies see us as a common Baltic market: however, we are three different countries, each with a local legislation, language and country-specific requirements – which means the each country also needs country-specific regulatory and pharmacovigilance expertise.
Medfiles has Baltic offices in Tartu in Estonia, Riga in Latvia and Kaunas in Lithuania, with 14 native-speaking professionals assisting our clients daily. Our Baltic team has a diverse combination of different backgrounds and educations: the team consists of pharmacists, medical doctors, medical attendants, a psychologist, a dentist, chemical and biotechnologies experts, a physiotherapist and a public health specialist.
The overall average regulatory affairs experience in the Baltic team is 18 years, and the skillset our team members have gathered from previous employments, such as working in the pharmaceutical industry and production, in a state agency or as a medical representative, quality manager or doctor, is a valuable asset both for the team and for our clients. We all work together as one team, complementing each other’s professional expertise, to provide the most efficient solutions to all our clients.
Our people are very familiar with the local legislation of each of our countries as well as the general EU legislation and can navigate smoothly in this complex regulatory field. In our daily work, we assist our clients in many regulatory affairs and local pharmacovigilance areas, supporting them in a wide variety of questions, tasks and projects related to different product groups, such as human and veterinary medicinal products, medical devices, cosmetics, food supplements and other food products.
Our most frequently assigned tasks include:
- Registration and license maintenance activities (NP, MRP/DCP, CP) of medicinal products, eCTD submissions
- Local PV activities
- Registration and notification activities for other products, such as food supplements and medical devices
- Compliance review of product information
- Compliance review of marketing materials and promotional materials
- Support in creating package or labelling materials
- Consultation regarding local requirements, including providing valuable advice based on previous experience
- Communication with local authorities
- Translations from English to our local languages Estonian, Latvian and Lithuanian
- Price and reimbursement activities.
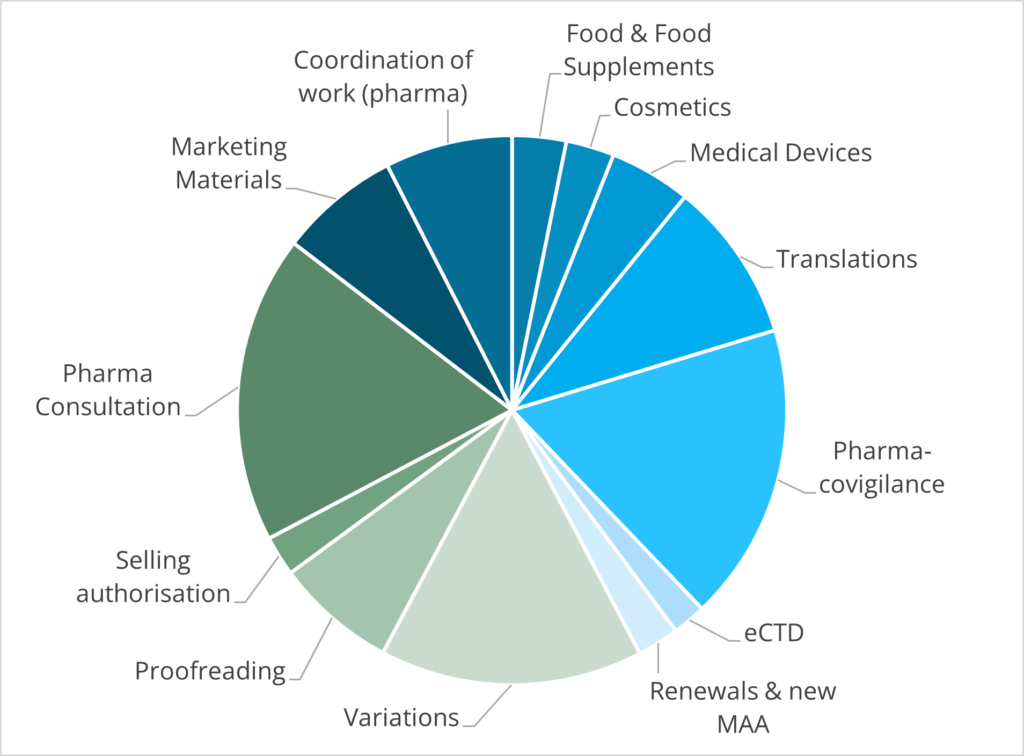
The chart below shows how the tasks in our team were divided in 2023.
We are trusted partner providing tailored and flexible top-quality service, with our 25 years of experience on the market and support from Medfiles Group. Three Baltic countries, one Medfiles Baltic team at your service!


