Clinical study services for food products
Clinical studies with foods are done to substantiate health claim dossiers or novel food applications according to the requirements of the European Food Safety Authority EFSA or the United States Food and Drug Administration FDA. Medfiles performs intervention studies with finished food products and food ingredients, as well as so-called proof of concept or pilot studies that support the R&D process of new ingredients. Studies are always conducted according to the applicable GCP requirements.
We offer comprehesive service packages from study planning to reporting or single services from our flexible service selection.
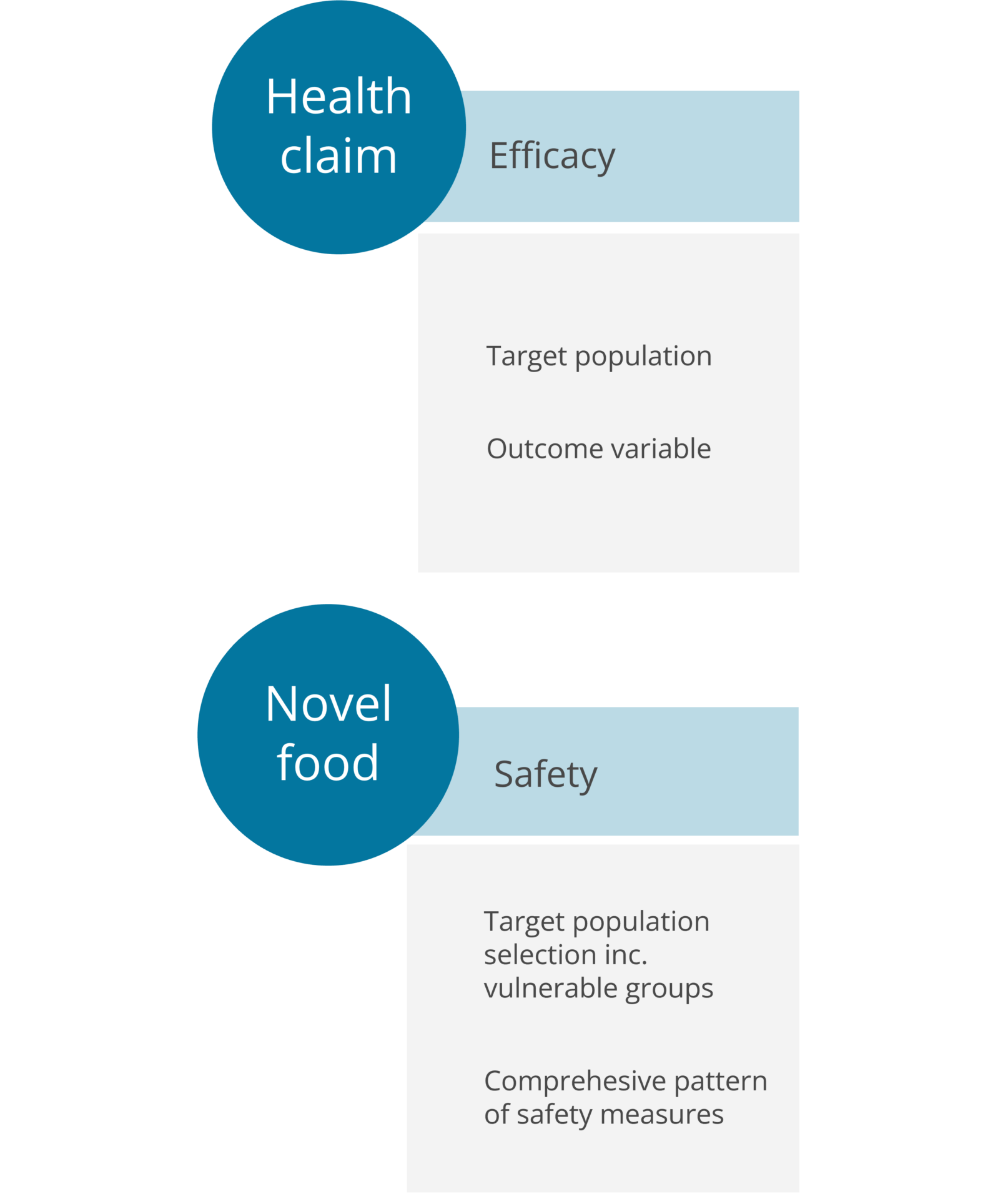
Food interventions for health claims
Medfiles has extensive experience in planning clinical studies. We see to that the efficacy documentation meets the expectations of the authorities when the objective is to achieve a health claim approval in the EU. The process starts with our regulatory expert team deciding the wording of the health claim, which is often done after a gap analysis of the existing documentation. After this, our clinical team works in close collaboration with the regulatory experts to define suitable outcome variables and the appropriate study design to meet the ultimate goal, the approval of the health claim.
Food interventions for novel foods and GRAS (Generally Recognized As Safe) ingredients
For novel foods and new food ingredients, the EU market approval often requires human safety data, for instance for the novel food process. Medfiles conducts clinical trials on food ingredients to demonsrate the safety of food products in the short and long-term.
Particulars in dietary interventions
Once a diet is intervened, it is of utmost importance to collect data on the use of the test food product, but also on any other changes in the diet. Medfiles with its qualified nutrition experts can plan and perform dietary monitoring, including the appropriate records, and thereafter make the required nutrient intake calculations. See also:
EXPERIENCE – clinical studies for Food products
Medfiles has a proven track record of multiple dietary interventions with various types of food in various health areas. Our permanent professional site partners can perform even the most demanding nutrition interventions.
Foods tested
- Cereals
- Snacks
- Drinks
- Comprehensive diet modifications
Ingredients tested
- Fibers
- Lipid ingredients
- Bioactive peptides
- Probiotics
- Polyphenols
Health areas
- Safety
- Cardiovascular risk factors
- Glucose metabolism
- Cognitive functions
- Skin health
clinical team leaders

Essi Sarkkinen
Director, Development & Operations
Essi Sarkkinen has worked at Medfiles since 2014, first leading the Food & Feed and Cosmetics Unit and for the past five years, leading the Medfiles Clinical Unit having experts in Finland and the Baltics. In the beginning of 2024, she was nominated as a Director of Development & Operations.
Essi has a PhD in clinical nutrition and she is adjunct professor at the University of Eastern Finland. Essi has published dozens of scientific articles in peer-reviewed journals and supervised four academic dissertations. After her academic career, she has worked for over 15 years managing and leading contract research in the field of food and nutrition. In the beginning of the 2000s, Essi started working with clinical trials for medicinal products and good clinical practice (GCP), as well as regulatory affairs concerning food products, including work with health claims and novel foods.
Essi has over 30 years of experience in clinical trials and medical writing as well as know-how of R&D and regulatory affairs in the fields of food, medical devices and pharmaceuticals. All in all, she has worked in various leadership positions in contract research organisations for over 25 years.

Satu Koskimies
Head of Clinical Operations
Satu Koskimies has worked at Medfiles since 2022, first as a clinical project manager and from the beginning of 2023, as Head of Clinical Operations. Satu has a M.Sc. in biology, and she is also a practical paediatric nurse and a seasoned clinical trial professional.
Since 2001, Satu has accumulated more than two decades of experience in clinical project management and coordination, medical writing and regulatory intelligence.
At Medfiles, she is involved with both medicinal products and medical devices, with a history of previously working with various CROs and biotech and pharma companies. Actively participating in the creation and implementation of robust quality management systems, preparing new operating procedures and enhancing high-quality performance in clinical research activities have been her favourite assignments during her career.
As Head of Clinical Operations, Satu supports and leads the Clinical Team at Medfiles, consisting of skilled, competent and dedicated clinical project managers and clinical research associates.
