Clinical investigation and performance evaluation services for medical devices
Medfiles manages clinical investigations and clinical performance studies from site and investigator qualifications to report preparation. Since the Medical Device Regulation and In vitro Diagnostic Medical Devices Regulation have been taken into use, the requirements have become much more demanding than before, which means that more and more companies are obliged to perform clinical investigations. As a result of this, Medfiles‘ experience in managing medical device investigations has increased significantly in the recent years.
In addition to project coordination and monitoring, our clinical project managers and CRAs can provide training to the sponsors regarding regulatory requirements and applicable standards, in order to successfully execute the clinical investigation or study.
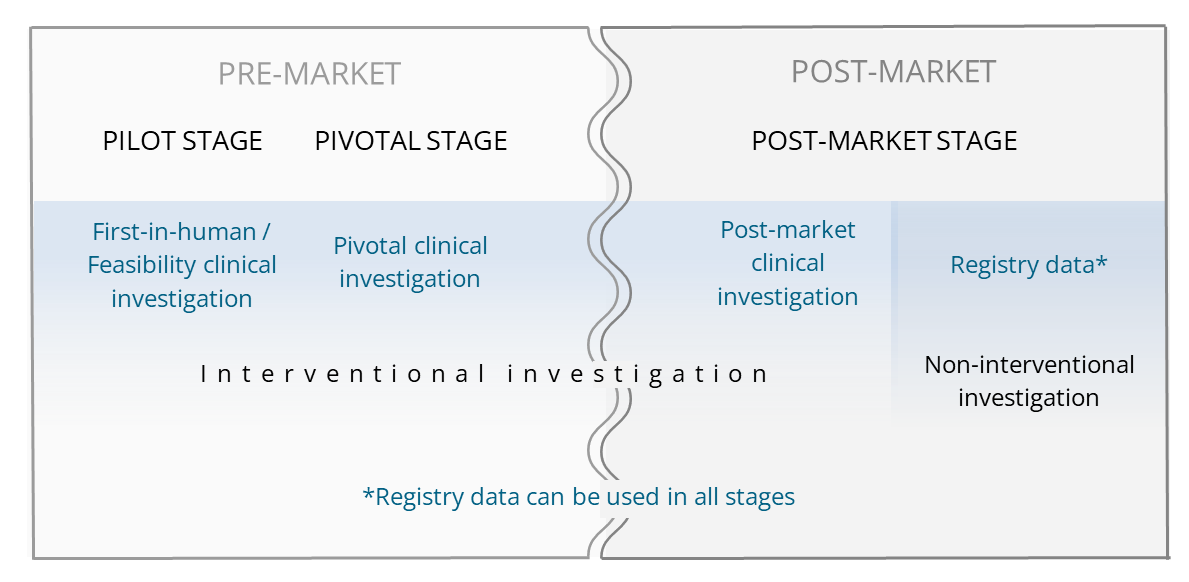
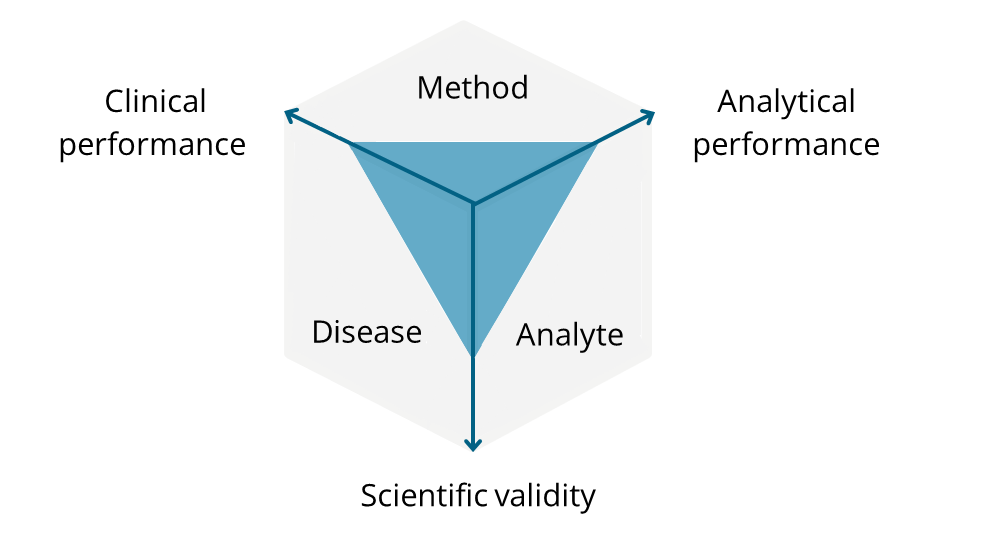
Our in-depth knowledge of medical devices ensures that your investigations and studies meet the recent requirements for clinical investigations and performance studies set forth in the ISO 14155 and ISO 20916 standards. Our dedicated SOPs for clinical investigations of medical devices further ensure the quality and compliant conduct of your studies. See also:
Free Guide: HOW TO MEET MDR GOOD CLINICAL PRACTICE REQUIREMENTS IN PRACTICE?
Do you work with medical devices and need information on the MDR/ISO 14155:2020 good clinical practice requirements? The MDR states that clinical investigations must be performed according to good clinical practice. In addition to observing the requirements set in the MDR itself, sponsors must also be aware of the requirements set in the ISO standard. We have created a guide on what you need to know about these requirements regarding the clinical trial set-up, monitoring and ending phase.

WellO2 & Medfiles: good collaboration smooths the path to clinical study success
“Collaboration with Medfiles was smooth in all respects. We felt that our questions were always welcome, and we always received clear and practical guidance, helping us understand what even the things that sounded difficult meant in practice and what we should do in different situations. We were always guided in the right direction along the research path, which ensured successful completion of the investigation.”
EXPERIENCE – Clinical investigations for medical devices
We provide continuous training for our employees and carefully follow the development of regulations to ensure the highest quality and compliance of our services. Our core competence is in clinical investigations on medical devices.
Indications
- Respiratory
- Wound care
- Orthopedic surgery
- Cancer early detection
Type of devices
- Class I, IIa, IIb and III
- Invasive/non-invasive
- Biogradable
- IVD
- Hematology
- Cancer
clinical team leaders

Essi Sarkkinen
Director, Development & Operations
Essi Sarkkinen has worked at Medfiles since 2014, first leading the Food & Feed and Cosmetics Unit and for the past five years, leading the Medfiles Clinical Unit having experts in Finland and the Baltics. In the beginning of 2024, she was nominated as a Director of Development & Operations.
Essi has a PhD in clinical nutrition and she is adjunct professor at the University of Eastern Finland. Essi has published dozens of scientific articles in peer-reviewed journals and supervised four academic dissertations. After her academic career, she has worked for over 15 years managing and leading contract research in the field of food and nutrition. In the beginning of the 2000s, Essi started working with clinical trials for medicinal products and good clinical practice (GCP), as well as regulatory affairs concerning food products, including work with health claims and novel foods.
Essi has over 30 years of experience in clinical trials and medical writing as well as know-how of R&D and regulatory affairs in the fields of food, medical devices and pharmaceuticals. All in all, she has worked in various leadership positions in contract research organisations for over 25 years.

Satu Koskimies
Head of Clinical Operations
Satu Koskimies has worked at Medfiles since 2022, first as a clinical project manager and from the beginning of 2023, as Head of Clinical Operations. Satu has a M.Sc. in biology, and she is also a practical paediatric nurse and a seasoned clinical trial professional.
Since 2001, Satu has accumulated more than two decades of experience in clinical project management and coordination, medical writing and regulatory intelligence.
At Medfiles, she is involved with both medicinal products and medical devices, with a history of previously working with various CROs and biotech and pharma companies. Actively participating in the creation and implementation of robust quality management systems, preparing new operating procedures and enhancing high-quality performance in clinical research activities have been her favourite assignments during her career.
As Head of Clinical Operations, Satu supports and leads the Clinical Team at Medfiles, consisting of skilled, competent and dedicated clinical project managers and clinical research associates.
